Overall study management
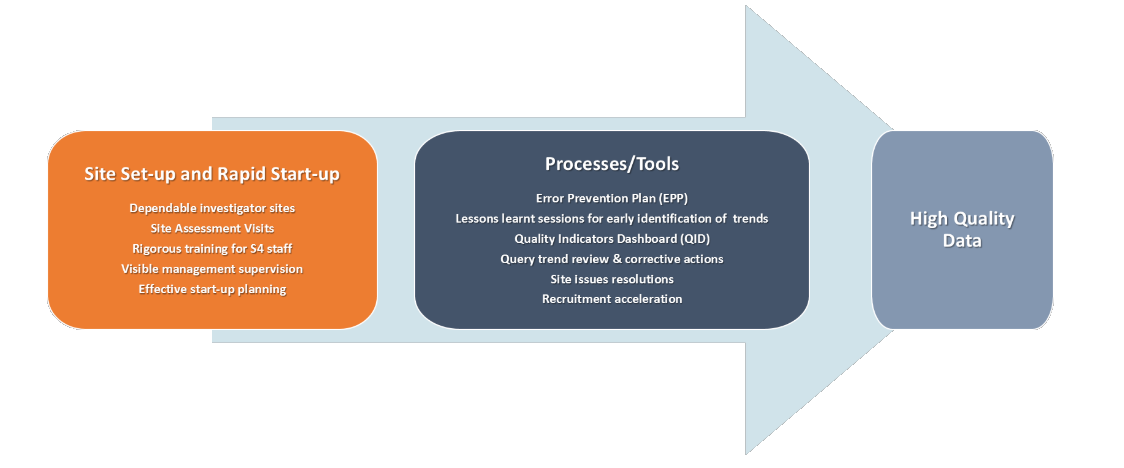
At KCR, we put a lot of attention and commitment to ensure the performance and quality of study deliverables. The Site Support model we implement is metrics-driven and it includes process and tools which integrates all key drivers for the successful conduct of your studies at sites.
With the vast experience in this field, KCR understands that efficient and rapid start-up planning is key to the success of any clinical trial. Therefore, we support the identification and selection of high quality and the most dependable investigator sites through our detailed and structured feasibility. KCR has agreements in place with many sites who have experienced investigators, suitable facilities and infrastructure as well as established processes for clinical trials.
We centrally organize and support simultaneous start-up activities for our sites through our experienced team of in-house project associates. This significantly reduces start-up timelines for KCR sites. These activities are as follows:
- Collection of essential documents for the regulatory submission and medicine release package
- Informed Consent Form (ICF) review and site-specific customization of ICFs
- Ethics Committee submission coordination
- Contract negotiation
- Adherence to the local country specific requirements and addressing study specific requirements at all the sites
We deploy well-trained, qualified, capable and dedicated site operations specialists and/or site coordinators at each Investigator site. These site specialists and/or coordinators provide support for the day to day study-related activities at the site and report centrally into our senior Project Managers at KCR.
In the advice with Investigators at the site and with our customer, KCR implements various metrics-driven processes and tools such as quality indicators dashboard, error prevention plan, lessons learned session, etc. These processes help to ensure speed-up patient recruitment, proper study conduct and overall high quality and credible patient data at its sites.

